
For the first time, MIT chemists have mapped out in detail how proton-coupled electron transfers happen at the surface of an electrode. Their results could help researchers to design more efficient fuel cells, batteries, or other energy technologies. Credit: MIT News, iStock
New insights into how proton-coupled electron transfers occur at an electrode could help researchers design more efficient fuel cells and electrolyzers.
A key chemical reaction — in which the movement of protons between the surface of an electrode and an electrolyte drives an electric current — is a critical step in many energy technologies, including fuel cells and the electrolyzers used to produce hydrogen gas.
For the first time,
“Our advance in this paper was studying and understanding the nature of how these electrons and protons couple at a surface site, which is relevant for catalytic reactions that are important in the context of energy conversion devices or catalytic reactions,” says Yogesh Surendranath, a professor of chemistry and chemical engineering at MIT and the senior author of the study.
Among their findings, the researchers were able to trace exactly how changes in the pH of the electrolyte solution surrounding an electrode affect the rate of proton motion and electron flow within the electrode.
MIT graduate student Noah Lewis is the lead author of the paper, which was published recently in Nature Chemistry. Ryan Bisbey, a former MIT postdoc; Karl Westendorff, an MIT graduate student; and Alexander Soudackov, a research scientist at 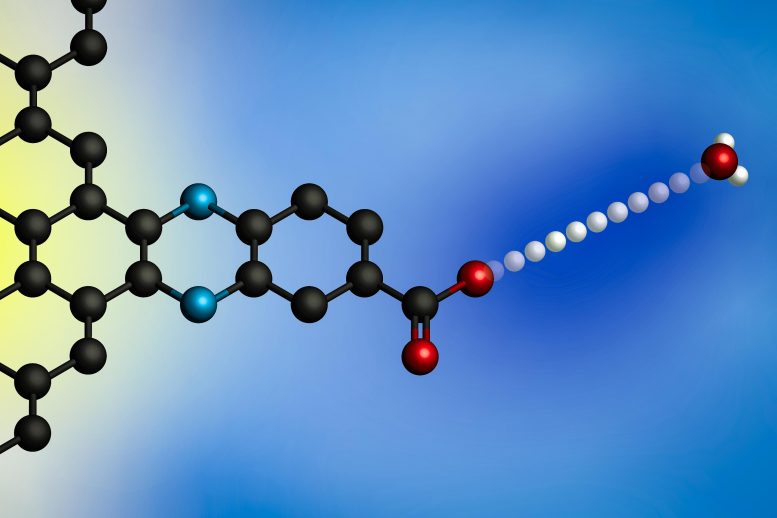
Applying an electric potential causes a proton to transfer from a hydronium ion (at right) to an electrode’s surface. Using electrodes with molecularly defined proton binding sites, MIT researchers developed a general model for these interfacial proton-coupled electron transfer reactions. Credit: Courtesy of the researchers