
Researchers at the University of California San Diego have developed a new cathode material for solid-state lithium-sulfur batteries that significantly improves their electrical conductivity and self-healing properties. This material addresses key challenges associated with sulfur cathodes, such as poor electron conductivity and structural damage during use. The innovation holds promise for doubling the energy density of batteries in electric vehicles without increasing weight and extends the battery life, making solid-state lithium-sulfur batteries a more viable and environmentally friendly option. Credit: David Baillot/UC San Diego Jacobs School of Engineering
Researchers have moved one step closer to making solid-state batteries from lithium and sulfur a practical reality. A team led by engineers at the University of California San Diego developed a new cathode material for solid-state lithium-sulfur batteries that is electrically conductive and structurally healable—features that overcome the limitations of these batteries’ current cathodes.
The work was recently published in the journal Nature.
Solid-state lithium-sulfur batteries are a type of rechargeable battery consisting of a solid electrolyte, an anode made of lithium metal, and a cathode made of sulfur. These batteries hold promise as a superior alternative to current lithium-ion batteries as they offer increased energy density and lower costs. They have the potential to store up to twice as much energy per kilogram as conventional lithium-ion batteries—in other words, they could double the range of electric vehicles without increasing the battery pack’s weight. Additionally, the use of abundant, easily sourced materials makes them an economically viable and environmentally friendlier choice.
Challenges and Breakthroughs
However, the development of lithium-sulfur solid-state batteries has been historically plagued by the inherent characteristics of sulfur cathodes. Not only is sulfur a poor electron conductor, but sulfur cathodes also experience significant expansion and contraction during charging and discharging, leading to structural damage and decreased contact with the solid electrolyte. These issues collectively diminish the cathode’s ability to transfer charge, compromising the overall performance and longevity of the solid-state battery.
To overcome these challenges, a team led by researchers at the UC San Diego Sustainable Power and Energy Center developed a new cathode material: a crystal composed of sulfur and iodine. By inserting iodine molecules into the crystalline sulfur structure, the researchers drastically increased the cathode material’s electrical conductivity by 11 orders of magnitude, making it 100 billion times more conductive than crystals made of sulfur alone.
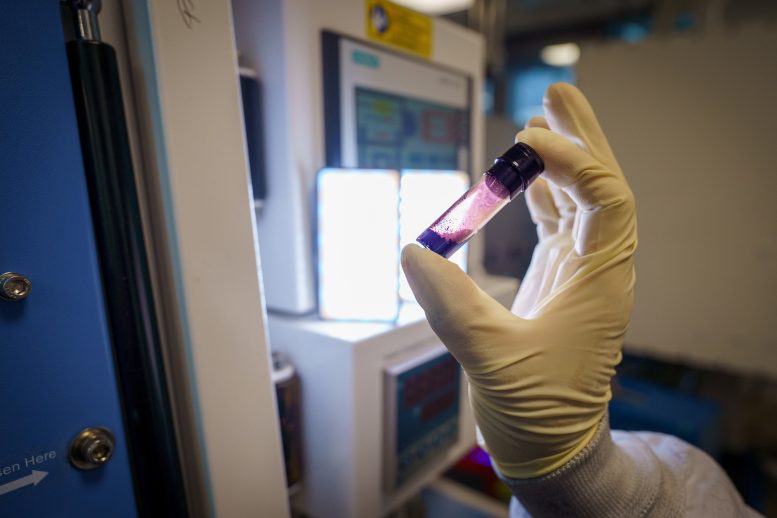
The cathode material heals by melting from a brown powder to a deep purple-red liquid. Credit: David Baillot/UC San Diego Jacobs School of Engineering
“We are very excited about the discovery of this new material,” said study co-senior author Ping Liu, a professor of nanoengineering and director of the Sustainable Power and Energy Center at UC San Diego. “The drastic increase in electrical conductivity in sulfur is a surprise and scientifically very interesting.”
Moreover, the new crystal material possesses a low melting point of 65 degrees SciTechDaily