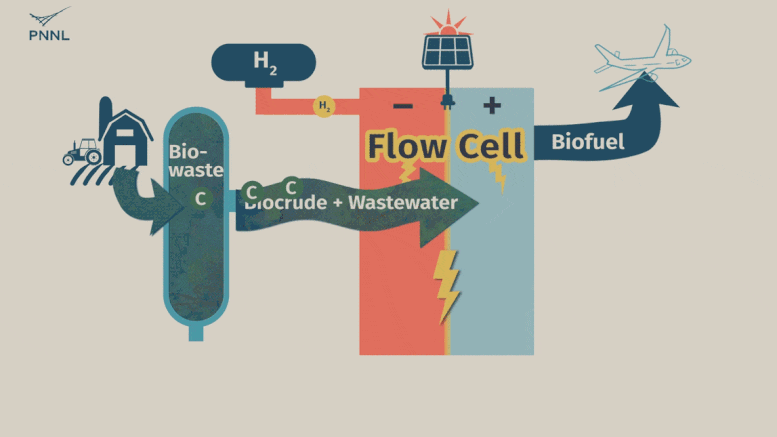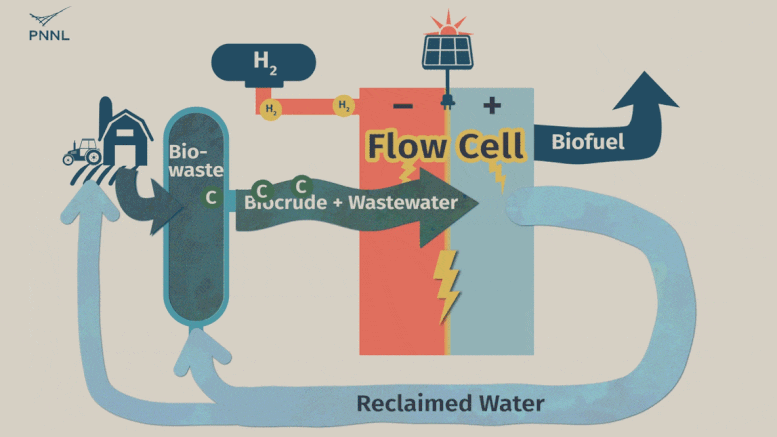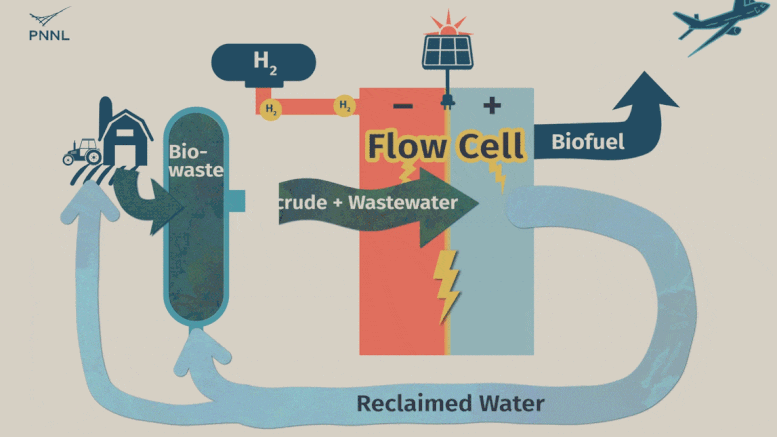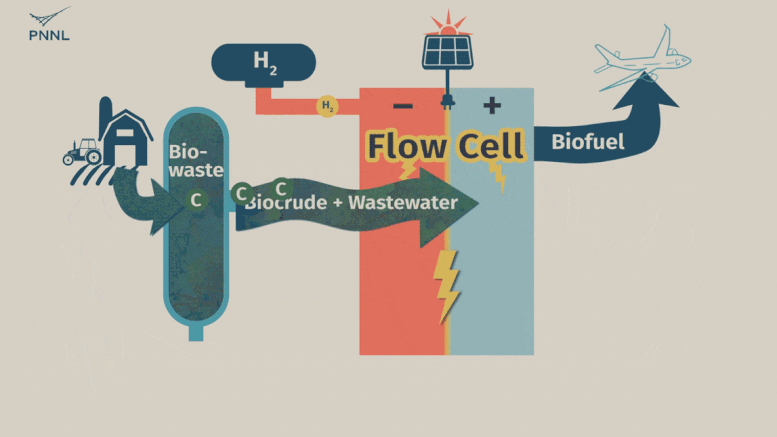
Waste carbon from farms, sewage and other sources can be processed into high-grade bio-based fuels more easily with a new PNNL-developed flow cell. In this animation, the flow cell receives biocrude and wastewater from a hydrothermal liquefaction process. It then removes carbon from wastewater, allowing the clean water to be reused. The system even generates hydrogen, a valuable fuel that can be captured, reducing the cost of the whole operation. Credit: Sara Levine | Pacific Northwest National Laboratory
Patent-pending process removes biofuel contaminants from wastewater using an additive-free process that generates hydrogen to fuel its own operation
The holy grail of biofuel researchers is to create a self-sustaining process that converts waste from sewage, food crops, algae, and other renewable carbon sources into fuels while keeping waste carbon out of our environment and water. Although much progress has been made in transforming such trash into usable fuel, completing the cycle with clean energy has proven to be a difficult nut to crack.
A team of researchers at the Department of Energy’s Pacific Northwest National Laboratory (PNNL) has now devised a system that accomplishes just that. The PNNL electrocatalytic oxidation fuel recovery system converts what was previously thought to be unrecoverable, diluted “waste” carbon into valuable chemicals while also producing useful hydrogen. Because renewable energy is used, the process is carbon-neutral or even possibly carbon-negative.
The key to making it all work is an elegantly designed catalyst that combines billions of infinitesimally small metal particles and an electric current to speed up the energy conversion at room temperature and pressure.

Juan A. Lopez-Ruiz, a PNNL chemical engineer, led a research team that recently developed a novel flow cell reactor that makes the path to renewable fuel easier. Credit: Andrea Starr | Pacific Northwest National Laboratory
“The currently used methods of treating biocrude requires high-pressure hydrogen, which is usually generated from natural gas,” said Juan A. Lopez-Ruiz, a PNNL chemical engineer and project lead. “Our system can generate that hydrogen itself while simultaneously treating the wastewater at near atmospheric conditions using excess renewable electricity, making it inexpensive to operate and potentially carbon neutral.”
A hungry system
The research group tested the system in the laboratory using a sample of wastewater from an industrial-scale biomass conversion process for over 200 hours of continuous operation without losing any efficiency in the process. The sole constraint was that the research team’s wastewater sample had run out.
“It’s a hungry system,” Lopez-Ruiz said. “The reaction rate of the process is proportional to how much waste carbon you are trying to convert. It could run indefinitely if you had wastewater to keep cycling through it.”
The patent-pending system solves several problems that have plagued efforts to make biomass an economically viable source of renewable energy, according to Lopez-Ruiz.
“We know how to turn biomass into fuel,” Lopez-Ruiz said. “But we still struggle to make the process energy-efficient, economical, and environmentally sustainable—especially for small, distributed scales. This system runs on electricity, which can come from renewable sources. And it generates its own heat and fuel to keep it running. It has the potential to complete the energy recovery cycle.”
“As the electric grid starts to shift its energy sources toward integrating more renewables,” he added, “it makes more and more sense to rely on electricity for our energy needs. We developed a process that uses electricity to power conversion of carbon compounds in wastewater into useful products while removing impurities like nitrogen and sulfur compounds.”
Closing the energy gap
Hydrothermal liquefaction (HTL) is a very efficient method for converting wet waste carbon to fuel. This process, in essence, shortens the time required to produce natural fossil fuels by turning wet biomass into energy-dense biocrude oil in hours rather than millennia. However, the process is incomplete in the sense that the wastewater generated as part of the process requires further treatment in order to get added value from what would otherwise be a liability.
“We realized that same (electro)chemical reaction that removed the organic molecules from wastewater could be also used to directly upgrade the biocrude at room temperature and atmospheric pressure as well,” Lopez-Ruiz said.
This is where the new PNNL process comes into play. Unrefined biocrude and wastewater can be fed into the system directly from an HTL output stream or other wet waste. The PNNL process consists of what’s called a flow cell where the wastewater and biocrude flows through the cell and encounters a charged environment created by an electric current. The cell itself is divided in half by a membrane.

A new patent-pending flow cell bioreactor developed at Pacific Northwest National Laboratory can purify wastewater (seen here) and generate hydrogen to help fuel the process. Credit: Andrea Starr | Pacific Northwest National Laboratory
The positively charged half, called an anode, contains a thin titanium foil coated with nanoparticles of ruthenium oxide. Here, the waste stream undergoes a catalytic conversion, with biocrude being converted to useful oils and paraffin. Simultaneously, water-soluble contaminants, such as oxygen and nitrogen-containing compounds, undergo a chemical conversion that turns them into nitrogen and oxygen gasses—normal components of the atmosphere. The wastewater that emerges from the system, with contaminants removed, can then be fed back into the HTL process.
On the negatively charged half of the flow cell, called a cathode, a different reaction takes place that can either hydrogenate organic molecules (such as the ones in treated biocrude) or generate hydrogen gas—an emerging energy source that the flow cell developers see as a potential source of fuel.
“We see the hydrogen byproduct generated by the process as a net plus. When collected and fed into the system as a fuel, it could keep the system running with fewer energy inputs, potentially making it more economical and carbon-neutral than current biomass conversion operations,” said Lopez-Ruiz.
The speed of chemical conversion provides an added benefit to the system.
“We did a comparison of rates—that is how fast we can remove oxygen from organic molecules with our system as opposed to the energy-intensive thermal removal,” Lopez-Ruiz said. “We obtained more than 100 times higher conversion rates with the electrochemical system at atmospheric conditions than with the thermal system at intermediate hydrogen pressures and temperatures.” These findings were published in the Journal of Applied Catalysis B: Environmental in November 2020.
Reducing rare Earth metal use
One significant disadvantage of many commercial technologies is their dependency on rare Earth metals, sometimes referred to as platinum group metals. The worldwide supply chain for these elements is mainly reliant on outdated extraction technologies that are energy-intensive, use enormous amounts of water, and generate hazardous waste. According to the Department of Energy, which has made domestic supply a primary priority, imports account for 100 percent of the United States’ supply for 14 of 35 critical materials and more than half of 17 others.
The system addresses this problem by incorporating a unique method of depositing nanoparticles of the metals responsible for the chemical conversion. These particles have a large surface area, which requires less metal to do its work. “We found that using metal nanoparticles as opposed to metal thin films and foils reduced the metal content and improved the electrochemical performance,” said Lopez-Ruiz. These findings were recently published in the Journal of Applied Catalysis B: Environmental. The novel catalyst requires 1,000 times less precious metal, in this case ruthenium, than is commonly needed for similar processes. Specifically, the laboratory-scale flow reactor uses an electrode with about 5 to 15 milligrams of ruthenium, compared with about 10 grams of platinum for a comparable reactor.
About those useless carbon compounds
The research team has also shown that the PNNL process can handle the processing of small water-soluble carbon compounds—byproducts found in the water waste stream of current HTL processes—as well as many other industrial processes. There are about a dozen of these devilishly difficult to process small, carbon compounds in the wastewater streams at low concentrations. Until now, there has been no cost-effective technology to handle them. These short-chain carbon compounds, like propanoic
“We at CogniTek are excited by the opportunity to extend the PNNL technology, in combination with our core patents and patent pending decarbonization technology,” said CogniTek Chief Executive Officer Michael Gurin.
The technology, dubbed Clean Sustainable Electrochemical Treatment—or CleanSET, is available for license by other companies or municipalities interested in developing it for industry-specific uses in municipal wastewater treatment plants, dairy farms, breweries, chemical manufacturers and food and beverage producers. To learn more about how this technology works, or to schedule a meeting with a technology commercialization manager, visit PNNL’s Available Technologies site.
In addition to Lopez-Ruiz, the PNNL research team included Yang Qiu, Evan Andrews, Oliver Gutiérrez and Jamie Holladay. The research was supported by the Department of Energy’s Advanced Manufacturing Office and the Chemical Transformation Initiative, a Laboratory Directed Research and Development Program at PNNL. Portions of the research were conducted as part of a Cooperative Research and Development Agreement with Southern California Gas Company.
References: “Anodic electrocatalytic conversion of carboxylic acids on thin films of RuO2, IrO2, and Pt” by Yang Qiu, Juan A. Lopez-Ruiz, Udishnu Sanyal, Evan Andrews, Oliver Y. Gutiérrez and Jamie D. Holladay, 25 June 2020, Applied Catalysis B: Environmental.DOI: 10.1016/j.apcatb.2020.119277
“Electrocatalytic valorization into H2 and hydrocarbons of an aqueous stream derived from hydrothermal liquefaction” by Juan A. Lopez-Ruiz, Yang Qiu, Evan Andrews, Oliver Y. Gutiérrez and Jamie D. Holladay, 9 July 2020, Journal of Applied Electrochemistry.DOI: 10.1007/s10800-020-01452-x
“Electrocatalytic decarboxylation of carboxylic acids over RuO2 and Pt nanoparticles” by Yang Qiu, Juan A. Lopez-Ruiza, Guomin Zhu, Mark H. Engelhard, Oliver Y. Gutiérrez and Jamie D. Holladay, 1 January 2022, Applied Catalysis B: Environmental.DOI: 10.1016/j.apcatb.2021.121060
Source: SciTechDaily
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.