Researchers have made a significant advancement in polymer solar cell technology by developing a method to improve molecular interactions using side-chain engineering. This approach eliminates the need for toxic halogenated processing solvents, thereby enhancing the cells’ efficiency and stability. The study, which highlights the benefits of oligoethylene glycol (OEG)-based side chains, marks a crucial step towards more environmentally friendly and efficient solar cells suitable for wearable devices. Credit: SciTechDaily
Polymer solar cells, known for their light weight and flexibility, are ideal for wearable devices. Yet, their broader use is hindered by the toxic halogenated solvents required in their production. These solvents pose environmental and health risks, limiting the appeal of these solar cells. Alternative solvents, which are less toxic, unfortunately, lack the same solubility, necessitating higher temperatures and prolonged processing times.
This inefficiency further impedes the adoption of polymer solar cells. Developing a method to eliminate the need for halogenated solvents could significantly enhance the efficiency of organic solar cells, making them more suitable for wearable technology.
In a recently published paper, researchers outline how improving molecular interactions between the polymer donors and the small molecule acceptors using side-chain engineering can reduce the need for halogenated processing solvents.
The paper was recently published in Nano Research Energy.
“Blend morphology of polymer donors and small molecule acceptors are highly affected by their molecular interactions, which can be determined by interfacial energies between the donor and acceptor materials. When their surface tension values are similar, the interfacial energies and molecular interactions between the donors and the acceptors are expected to be more favorable,” said Yun-Hi Kim, a professor at Gyeongsang National University in Jinju, Republic of Korea. “To enhance the hydrophilicity of the polymer donors and reduce molecular demixing, side-chain engineering can be a plausible avenue.”
The Role of Side-Chain Engineering
Side-chain engineering is when a chemical group, called a side chain, is added to the main chain of a molecule. The chemical groups in the side chain affect the properties of the larger molecule. Researchers theorized that adding oligoethylene glycol (OEG)-based side chains would improve the hydrophilicity of the polymer donors thanks to the oxygen atoms in the side chains. A molecule with hydrophilicity is attracted to water.
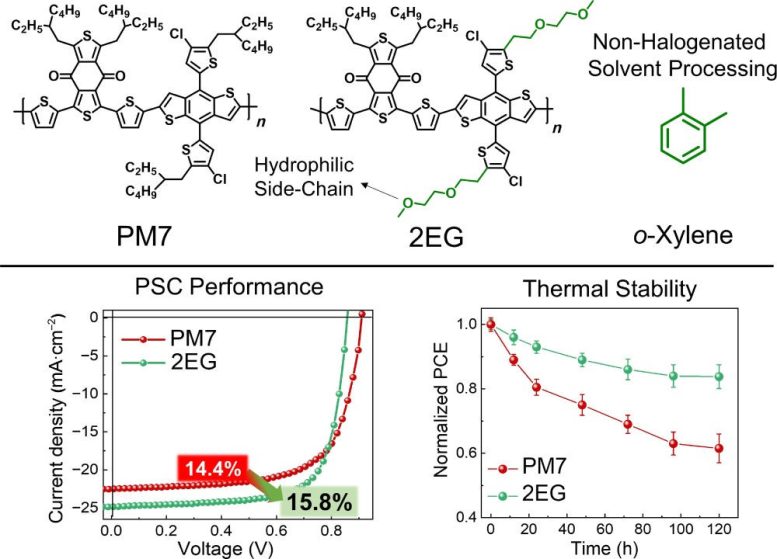
A blend of hydrocarbon and hydrophilic oligoethylene glycol (2EG) performed better than the standard solvent when used in PSC creation, based on overall performance and thermal stability. Credit: Nano Research Energy, Tsinghua University Press
Differences in the hydrophilicity of the polymer donors and the small molecule acceptors can impact how they interact. With increased hydrophilicity of the polymer donors and improved interactions between them and the small molecule acceptors, non-halogenated processing solvents can be used without sacrificing the performance of the solar cell. In fact, polymer solar cells made with OEG-based side chains attached to a benzodithiophene-based polymer donor had a higher power conversion efficiency at 17.7% compared to 15.6%.
Enhanced Efficiency and Stability
In order to compare results, researchers designed benzodithiophene-based polymer donors with either an OEG side chain, hydrocarbon side chains, or side chains that were 50% hydrocarbon and 50% OEG. “This elucidated the effect of side-chain engineering on blend morphology and performance of non-halogenated solvent-processed polymer solar cells,” said Kim. “Our findings demonstrate that polymers with hydrophilic OEG side chains can enhance the miscibility with small molecule acceptors and improve power conversion efficiency and device stability of polymer solar cells during non-halogenated processing.”
In addition to improved power conversion efficiency, the polymer solar cells with the OEG-based side chains had enhanced thermal stability. Thermal stability is essential for scaling polymer solar cells, so researchers heated them to 120 degrees SciTechDaily