The fusion of deuterium (D) and tritium (T) atoms has been proven in the laboratory to produce the highest energy gain at the ”lowest” temperatures. Credit: ITER
Fusion energy has the potential to supply safe, clean, and nearly limitless power. Although fusion reactions can occur for light nuclei weighting less than iron, most elements will not fuse unless they are in the interior of a star. To create burning plasmas in experimental fusion power reactors such as tokamaks and stellarators, scientists seek a fuel that is relatively easy to produce, store, and bring to fusion. The current best bet for fusion reactors is deuterium-tritium fuel. This fuel reaches fusion conditions at lower temperatures compared to other elements and releases more energy than other fusion reactions.
Deuterium and tritium are isotopes of hydrogen, the most abundant element in the universe. Whereas all isotopes of hydrogen have one proton, deuterium also has one neutron and tritium has two neutrons, so their ion masses are heavier than protium, the isotope of hydrogen with no neutrons. When deuterium and tritium fuse, they create a helium nucleus, which has two protons and two neutrons. The reaction releases an energetic neutron. Fusion power plants would convert energy released from fusion reactions into electricity to power our homes, businesses, and other needs.
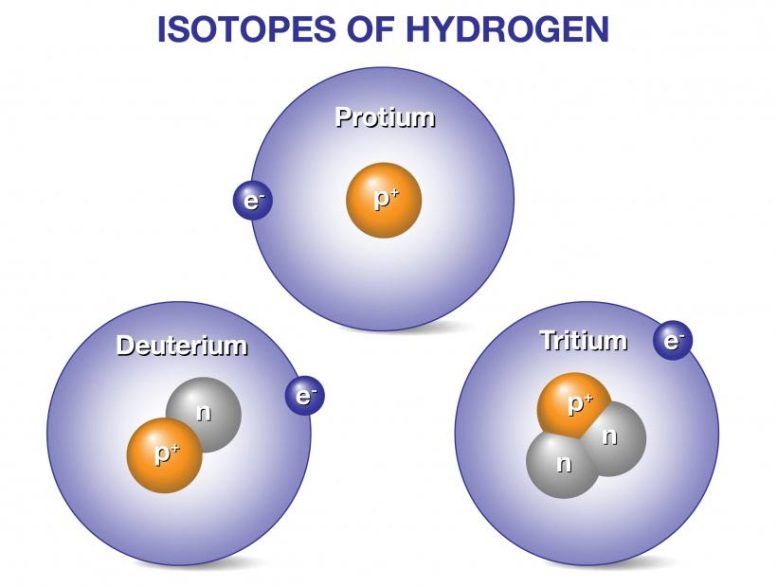
There is only one proton in the nucleus of all isotopes of hydrogen, but the number of neutrons varies. Credit: Image courtesy of General Atomics
Fortunately, deuterium is common. About 1 out of every 5,000 hydrogen atoms in seawater is in the form of deuterium. This means our oceans contain many tons of deuterium. When fusion power becomes a reality, just one gallon of seawater could produce as much energy as 300 gallons of gasoline.
Tritium is a radioactive isotope that decays relatively quickly (it has a 12-year half-life) and is rare in nature. Fortunately, exposing the more abundant element of lithium to energetic neutrons can generate tritium. A working fusion power plant would need enriched lithium to breed the tritium it needs to close the deuterium-tritium fuel cycle. Current R&D efforts are focused on advanced designs of tritium breeding blankets using lithium originally obtained from Earth based sources.
Deuterium-Tritium Fuel Facts
- Water made from deuterium is about 10 percent heavier than ordinary water. That’s why it is sometimes referred to as “heavy water.” It will actually sink to the bottom of a glass of ordinary water.
- Sources of tritium on Earth include natural production from interactions with cosmic rays, energy-producing nuclear fission reactors such as the heavy water CANDU reactor, and nuclear weapons testing.
- To avoid certain R&D challenges including structural material damage from energetic neutrons, fusion scientists are interested also in aneutronic fusion reactions (such as deuterium-helium-3 and proton-boron fusion) even though these fusion reactions occur at higher ion temperatures than for deuterium and tritium.
DOE Office of Science: Contributions to Deuterium-Tritium Fuel
Part of the mission of The Department of Energy Office of Science, Fusion Energy Sciences (FES) program is to develop a practical fusion energy source. FES works with the Advanced Scientific Computing Research program using scientific computing to advance fusion science and understand the effect of ion mass on various
smooth jazz