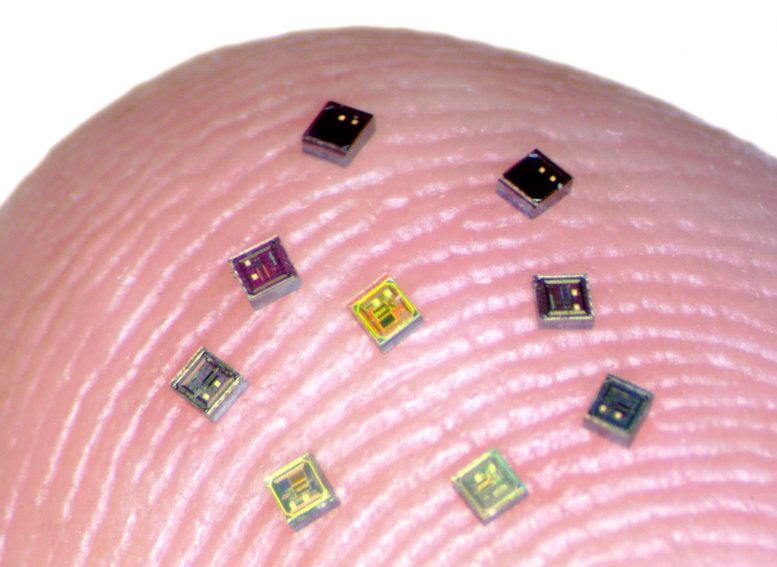
Tiny chips called neurograins are able to sense electrical activity in the brain and transmit that data wirelessly. Credit: Jihun Lee / Brown University
Brain-computer interfaces (BCIs) are emerging assistive devices that may one day help people with brain or spinal injuries to move or communicate. BCI systems depend on implantable sensors that record electrical signals in the brain and use those signals to drive external devices like computers or robotic prosthetics.
Most current BCI systems use one or two sensors to sample up to a few hundred neurons, but neuroscientists are interested in systems that are able to gather data from much larger groups of brain cells.
Now, a team of researchers has taken a key step toward a new concept for a future BCI system — one that employs a coordinated network of independent, wireless microscale neural sensors, each about the size of a grain of salt, to record and stimulate brain activity. The sensors, dubbed “neurograins,” independently record the electrical pulses made by firing neurons and send the signals wirelessly to a central hub, which coordinates and processes the signals.
In a study published on August 12, 2021, in Nature Electronics, the research team demonstrated the use of nearly 50 such autonomous neurograins to record neural activity in a rodent.
The results, the researchers say, are a step toward a system that could one day enable the recording of brain signals in unprecedented detail, leading to new insights into how the brain works and new therapies for people with brain or spinal injuries.
“One of the big challenges in the field of brain-computer interfaces is engineering ways of probing as many points in the brain as possible,” said Arto Nurmikko, a professor in Brown’s School of Engineering and the study’s senior author. “Up to now, most BCIs have been monolithic devices — a bit like little beds of needles. Our team’s idea was to break up that monolith into tiny sensors that could be distributed across the cerebral cortex. That’s what we’ve been able to demonstrate here.”
The team, which includes experts from Brown, Baylor University, University of California at San Diego and Qualcomm, began the work of developing the system about four years ago. The challenge was two-fold, said Nurmikko, who is affiliated with Brown’s Carney Institute for Brain Science. The first part required shrinking the complex electronics involved in detecting, amplifying and transmitting neural signals into the tiny silicon neurograin chips. The team first designed and simulated the electronics on a computer, and went through several fabrication iterations to develop operational chips.
The second challenge was developing the body-external communications hub that receives signals from those tiny chips. The device is a thin patch, about the size of a thumb print, that attaches to the scalp outside the skull. It works like a miniature cellular phone tower, employing a network protocol to coordinate the signals from the neurograins, each of which has its own network address. The patch also supplies power wirelessly to the neurograins, which are designed to operate using a minimal amount of electricity.
“This work was a true multidisciplinary challenge,” said Jihun Lee, a postdoctoral researcher at Brown and the study’s lead author. “We had to bring together expertise in electromagnetics, radio frequency communication, circuit design, fabrication and neuroscience to design and operate the neurograin system.”
The goal of this new study was to demonstrate that the system could record neural signals from a living brain — in this case, the brain of a rodent. The team placed 48 neurograins on the animal’s cerebral cortex, the outer layer of the brain, and successfully recorded characteristic neural signals associated with spontaneous brain activity.
The team also tested the devices’ ability to stimulate the brain as well as record from it. Stimulation is done with tiny electrical pulses that can activate neural activity. The stimulation is driven by the same hub that coordinates neural recording and could one day restore brain function lost to illness or injury, researchers hope.
The size of the animal’s brain limited the team to 48 neurograins for this study, but the data suggest that the current configuration of the system could support up to 770. Ultimately, the team envisions scaling up to many thousands of neurograins, which would provide a currently unattainable picture of brain activity.
“It was a challenging endeavor, as the system demands simultaneous wireless power transfer and networking at the mega-bit-per-second rate, and this has to be accomplished under extremely tight silicon area and power constraints,” said Vincent Leung, an associate professor in the Department of Electrical and Computer Engineering at Baylor. “Our team pushed the envelope for distributed neural implants.”
There’s much more work to be done to make that complete system a reality, but researchers said this study represents a key step in that direction.
“Our hope is that we can ultimately develop a system that provides new scientific insights into the brain and new therapies that can help people affected by devastating injuries,” Nurmikko said.
Reference: “Neural recording and stimulation using wireless networks of microimplants” by Jihun Lee, Vincent Leung, Ah-Hyoung Lee, Jiannan Huang, Peter Asbeck, Patrick P. Mercier, Stephen Shellhammer, Lawrence Larson, Farah Laiwalla and Arto Nurmikko, 12 August 2021, Nature Electronics.DOI: 10.1038/s41928-021-00631-8
Other co-authors on the research were Ah-Hyoung Lee (Brown), Jiannan Huang (UCSD), Peter Asbeck (UCSD), Patrick P. Mercier (UCSD), Stephen Shellhammer (Qualcomm), Lawrence Larson (Brown) and Farah Laiwalla (Brown). The research was supported by the Defense Advanced Research Projects Agency (N66001-17-C-4013).