Researchers have spent more than three decades developing and studying miniature biosensors that can identify single molecules. In five to 10 years, when such devices may become a staple in doctors’ offices, they could detect molecular markers for cancer and other diseases and assess the effectiveness of drug treatment to fight those illnesses.
To help make that happen and to boost the accuracy and speed of these measurements, scientists must find ways to better understand how molecules interact with these sensors. Researchers from the National Institute of Standards and Technology (NIST) and Virginia Commonwealth University (VCU) have now developed a new approach. They reported their findings in a recent issue of Science Advances.
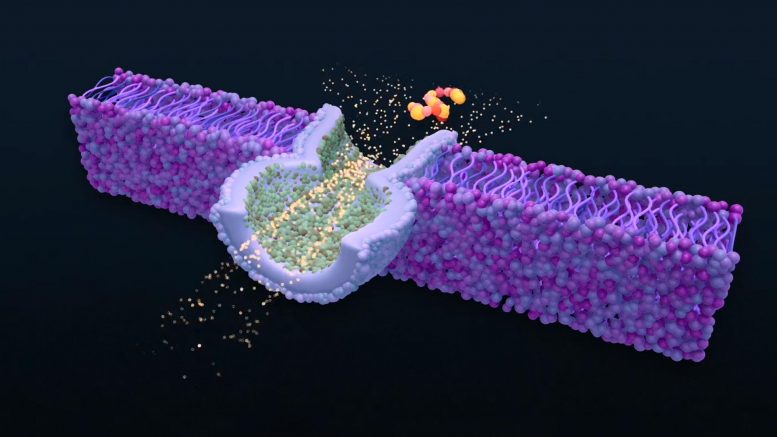
The team built its biosensor by making an artificial version of the biological material that forms a cell membrane. Known as a lipid bilayer, it contains a tiny pore, about 2 nanometers (billionths of a meter) wide in diameter, surrounded by fluid. Ions that are dissolved in the fluid pass through the nanopore, generating a small electric current. However, when a molecule of interest is driven into the membrane, it partially blocks the flow of current. The duration and magnitude of this blockade serve as a fingerprint, identifying the size and properties of a specific molecule.
To identify molecules, scientists can use a type of biosensor called a nanopore — a tiny hole in a membrane that allows fluid to flow through it. When a molecule of interest is driven into the pore, it partially blocks the flow of current, providing a signal researchers can use to identify the molecule. But in order to get a good measurement, the molecule must sit inside the pore for long enough. NIST researchers are using laser light to measure the energy of molecules as they transition into and out of nanopores. The resulting information can help scientists design optimized pores for detecting particular molecules. Credit: Sean Kelley/Inform Studio
To make accurate measurements for a large number of individual molecules, the molecules of interest must stay in the nanopore for an interval that is neither too long nor too short (the “Goldilocks” time), ranging from 100 millionths to 10 thousandths of a second. The problem is that most molecules only stay in the small volume of a nanopore for this time interval if the nanopore somehow holds them in place. This means that the nanopore environment must provide a certain barrier — for instance, the addition of an electrostatic force or a change in the nanopore’s shape — that makes it more difficult for the molecules to escape.
The minimum energy required to breach the barrier differs for each type of molecule and is critical for the biosensor to work efficiently and accurately. Calculating this quantity involves measuring several properties related to the energy of the molecule as it moves into and out of the pore.
Critically, the goal is to measure whether the interaction between the molecule and its environment arises primarily from a chemical bond or from the ability of the molecule to wiggle and move freely throughout the capture and release process.
Until now, reliable measurements to extract these energetic components have been missing for a number of technical reasons. In the new study, a team co-led by Joseph Robertson of NIST and Joseph Reiner of VCU demonstrated the ability to measure these energies with a rapid, laser-based heating method.
The measurements must be conducted at different temperatures, and the laser heating system ensures that these temperature changes occur rapidly and reproducibly. That enables researchers to complete measurements in less than 2 minutes, compared to the 30 minutes or more it would otherwise require.
“Without this new laser-based heating tool, our experience suggests that the measurements simply won’t be done; they would be too time consuming and costly,” said Robertson. “Essentially, we have developed a tool that may change the development pipeline for nanopore sensors to rapidly reduce the guesswork involved in sensor discovery,” he added.
Once the energy measurements are performed, they can help reveal how a molecule interacts with the nanopore. Scientists can then use this information to determine the best strategies for detecting molecules.
For example, consider a molecule that interacts with the nanopore primarily through chemical — essentially electrostatic — interactions. To achieve the Goldilocks capture time, the researchers experimented with modifying the nanopore so that its electrostatic attraction to the target molecule was neither too strong nor too weak.
With this goal in mind, the researchers demonstrated the method with two small peptides, short chains of compounds that form the building blocks of proteins. One of the peptides, angiotensin, stabilizes blood pressure. The other peptide, neurotensin, helps regulate dopamine, a neurotransmitter that influences mood and may also play a role in colorectal cancer. These molecules interact with nanopores primarily through electrostatic forces. The researchers inserted into the nanopore gold nanoparticles capped with a charged material that boosted the electrostatic interactions with the molecules.
The team also examined another molecule, polyethylene glycol, whose ability to move determines how much time it spends in the nanopore. Ordinarily, this molecule can wiggle, rotate and stretch freely, unencumbered by its environment. To increase the molecule’s residence time in the nanopore, the researchers altered the nanopore’s shape, making it more difficult for the molecule to squeeze through the tiny cavity and exit.
“We can exploit these changes to build a nanopore biosensor tailored to detecting specific molecules,” says Robertson. Ultimately, a research laboratory could employ such a biosensor to identify biological molecules of interest or a doctor’s office could use the device to identify markers for disease.
“Our measurements provide a blueprint for how we can modify the interactions of the pore, whether it be through geometry or chemistry, or some combination of both, to tailor a nanopore sensor for detecting specific molecules, counting small numbers of molecules, or both,” said Robertson.
Reference: “Laser-based temperature control to study the roles of entropy and enthalpy in polymer-nanopore interactions” by Christopher E. Angevine, Joseph W.F. Robertson, Amala Dass and Joseph E. Reiner, 21 April 2021, Science Advances.DOI: 10.1126/sciadv.abf5462