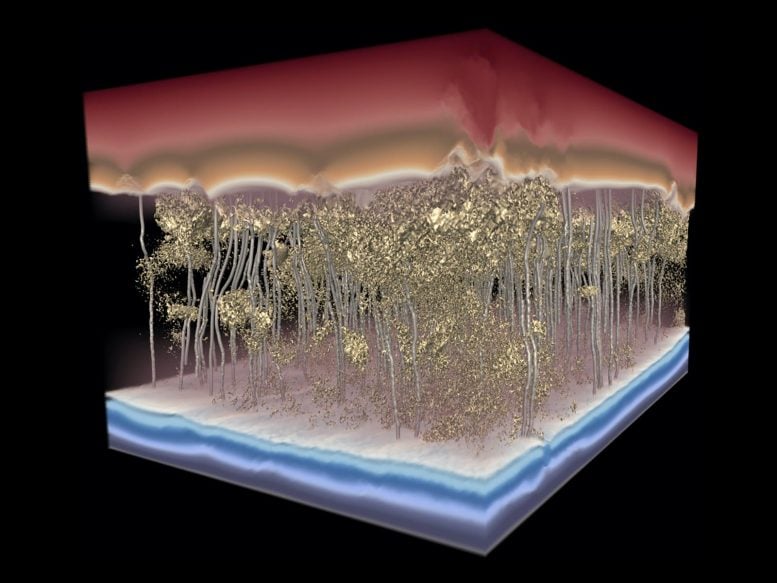
This 3D model of a polymer desalination membrane shows water flow — the silver channels, moving from top to bottom — avoiding dense spots in the membrane and slowing flow. Credit: Image by the Ganapathysubramanian research group/Iowa State University and Gregory Foss/Texas Advanced Computing Center
A desalination membrane acts as a filter for salty water: push the water through the membrane, get clean water suitable for agriculture, energy production and even drinking. The process seems simple enough, but it contains complex intricacies that have baffled scientists for decades — until now.
Researchers from Penn State, The University of Texas at Austin, Iowa State University, Dow Chemical Company and DuPont Water Solutions published a key finding in understanding how membranes actually filter minerals from water, online today (Dec. 31) in Science. The article will be featured on the print edition’s cover, to be issued tomorrow (Jan. 1).
“Despite their use for many years, there is much we don’t know about how water filtration membranes work,” said Enrique Gomez, professor of chemical engineering and materials science and engineering at Penn State, who led the research. “We found that how you control the density distribution of the membrane itself at the nanoscale is really important for water-production performance.”
Co-led by Manish Kumar, associate professor in the Department of Civil, Architectural and Environmental Engineering at UT Austin, the team used multimodal electron microscopy, which combines the atomic-scale detailed imaging with techniques that reveal chemical composition, to determine that desalination membranes are inconsistent in density and mass. The researchers mapped the density variations in polymer film in three dimensions with a spatial resolution of approximately one nanometer — that’s less than half the diameter of a DNA strand. According to Gomez, this technological advancement was key in understanding the role of density in membranes.
“You can see how some places are more or less dense in a coffee filter just by your eye,” Gomez said. “In filtration membranes, it looks even, but it’s not at the nanoscale, and how you control that mass distribution is really important for water-filtration performance.”
This was a surprise, Gomez and Kumar said, as it was previously thought that the thicker the membrane, the less water production. Filmtec, now a part of DuPont Water Solutions, which makes numerous desalination products, partnered with the researchers and funded the project because their in-house scientists found that thicker membranes were actually proving to be more permeable.
The researchers found that the thickness does not matter as much as avoiding highly dense nanoscale regions, or “dead zones.” In a sense, a more consistent density throughout the membrane is more important than thickness for maximizing water production, according to Gomez.
This understanding could increase membrane efficiency by 30% to 40%, according to the researchers, resulting in more water filtered with less energy — a potential cost-saving update to current desalination processes.
“Reverse osmosis membranes are so widely used for cleaning water, but there’s still a lot we don’t know about them,” Kumar said. “We couldn’t really say how water moves through them, so all the improvements over the last 40 years have essentially been done in the dark.”
Reverse osmosis membranes work by applying pressure on one side. The minerals stay there, while the water passes through. While more efficient than non-membrane desalination processes, this still takes an immense amount of energy, the researchers said, but improving the efficiency of the membranes could reduce that burden.
“Freshwater management is becoming a crucial challenge throughout the world,” Gomez said. “Shortages, droughts — with increasing severe weather patterns, it is expected this problem will become even more significant. It’s critically important to have clean water available, especially in low resource areas.”
The team continues to study the structure of the membranes, as well as the chemical reactions involved in the desalination process. They are also examining how to develop the best membranes for specific materials, such as sustainable yet tough membranes that can prevent the formation of bacterial growth.
“We’re continuing to push our techniques with more high-performance materials with the goal of elucidating the crucial factors of efficient filtration,” Gomez said.
More on this research:
- Desalination Breakthrough Maximizes Flow for Cheaper Water Filtration
- Nanoscale Control of Desalination Membranes Could Lead to Cheaper Water Filtration
Reference: “Nanoscale control of internal inhomogeneity enhances water transport in desalination membranes” by Tyler E. Culp, Biswajit Khara, Kaitlyn P. Brickey, Michael Geitner, Tawanda J. Zimudzi, Jeffrey D. Wilbur, Steven D. Jons, Abhishek Roy, Mou Paul, Baskar Ganapathysubramanian, Andrew L. Zydney, Manish Kumar and Enrique D. Gomez, 31 December 2020, Science.DOI: 10.1126/science.abb8518
Other contributors include first author Tyler E. Culp, Kaitlyn P. Brickey, Michael Geitner and Andrew Zydney, all of whom are affiliated with the Penn State Department of Chemical Engineering; Biswajit Khara and Baskar Ganapathysubramanian, both with the Department of Mechanical Engineering at Iowa State University; Tawanda J. Zimudzi of the Materials Research Institute (MRI) at Penn State; Jeffrey D. Wilbur and Steve Jons, both with DuPont Water Solutions; and Abhishek Roy and Mou Paul, both with Dow Chemical Company. Gomez is also affiliated with MRI. The microscopic work was conducted on electron microscopes in the Materials Characterization Lab in MRI. DuPont and the National Science Foundation funded the research.