In reservoir computing, information propagation is like rippling waves on the surface of a body of water; hence, the term “reservoir” is used. The underwater electrode pictured is the actual multi-terminal electrode used in this study. Credit: Megumi Akai-Kasaya et al.
Researchers led by Osaka University reveal the excellent information processing abilities of physical reservoirs based on electrochemical reactions in Faradic current and present a simple system for computing systems using electrochemical ion reactions.
After many decades of astonishing developments, advances in semiconductor-based computing are beginning to slow as transistors reach their physical limits in size and speed. However, the requirements for computing continue to grow, especially in artificial intelligence, where neural networks often have several millions of parameters. One solution to this problem is reservoir computing, and a team of researchers led by Osaka University, with colleagues from the University of Tokyo and
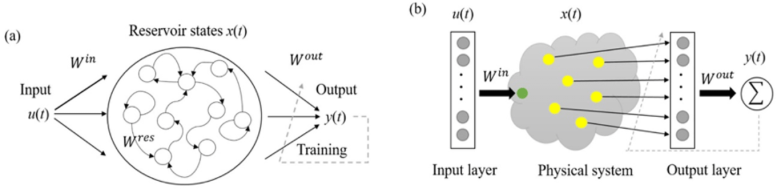
Physical reservoir computing and the construction of a molecular-based reservoir. (a) Structure of traditional reservoir computing. (b) Concept of our physical reservoir computing system. Credit: © 2022 Megumi Akai-Kasaya et al., Advanced Science
“Our simple testing device consists of 90 pairs of planar electrodes with an ionic solution dropped on its surface,” explains Professor Megumi Akai-Kasaya, lead author of the study. “The response voltage to the input voltage is then used as the response of the reservoir.” This voltage response is due to both the ionic currents that pass through the solution and the electrochemical current. This input–output relationship is both nonlinear and reproducible, which makes it suitable for use in reservoir computing. A unique multiway data acquisition system on the device controls the readout nodes, which enables parallel testing.
The researchers used the device to evaluate two liquids: polyoxometalate molecules in solution and deionized water. The system displayed a “feedforward connection” between nodes, regardless of which sample was used. However, there were differences. “The polyoxometalate solution increased the diversity of the response current, which makes it good at predicting periodic signals,” says Professor Akai-Kasaya. “But it turns out that deionized water is best for solving second-order nonlinear problems.” The good performance of these solutions demonstrates their potential for more complicated tasks, such as handwriting font recognition, isolated word recognition, and other classification tasks.
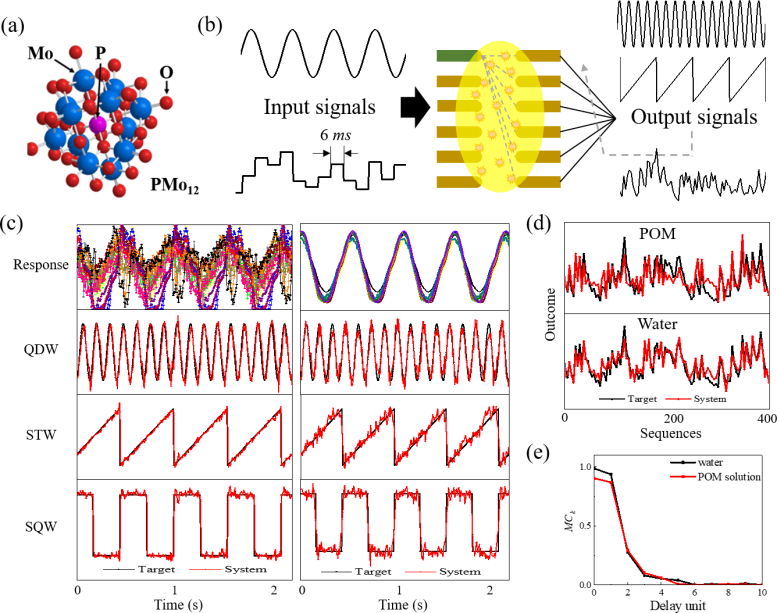
(a) Structure of the polyoxometalate (POM) molecule. (b) Schematic of the electrochemical-reaction-based reservoir. (c) Responses of the POM solution (left) and deionized water (right) to a sinusoidal signal and their prediction performances on a quadruple sine (QDW) target signal. (d) Prediction performances of the POM solution and water on a nonlinear target signal. Credit: © 2022 Megumi Akai-Kasaya et al., Advanced Science
The researchers believe that proton or ion transfer with minimal electrochemical reactions over short durations has the potential for development as a more computationally powerful computing system that is low in cost and energy-efficient. The simplicity of the proposed system opens up exciting new opportunities for developing computing systems based on electrochemical ion reactions.
Reference: “Physical Implementation of Reservoir Computing through Electrochemical Reaction” by Shaohua Kan, Kohei Nakajima, Tetsuya Asai and Megumi Akai-Kasaya, 29 December 2021, Advanced Science.DOI: 10.1002/advs.202104076